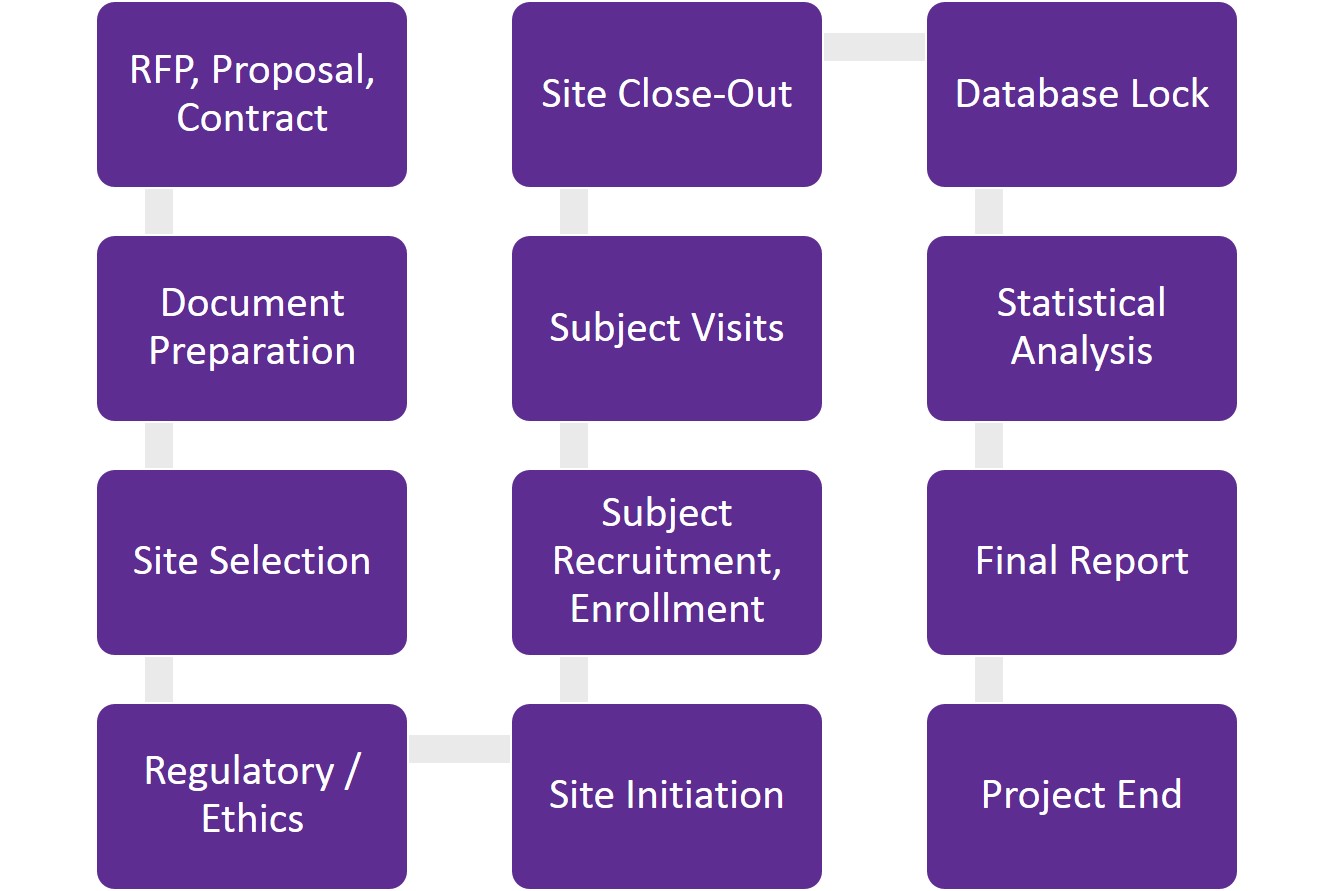
Study Close Out Visit Beaumont Health 04/04/2018 04/04/2018 Administrative Director Policy Clinical Research SOP's, Research Ins

Site Closeout Visit Readiness Checklist v20 1375 | PDF | Clinical Trial | Information Technology Management

CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure | Zenosis – Learning for Life